Stem cell therapies are at the forefront of modern medicine, offering hope for conditions ranging from degenerative diseases to sports injuries. But transforming a breakthrough in a laboratory into a treatment that patients can safely use in real-world settings is a complex journey. Understanding this pathway can give patients, caregivers, and healthcare professionals a clearer picture of how these therapies evolve from promising science to practical medical solutions.
From Discovery to Preclinical Research
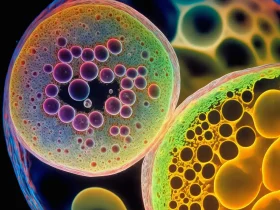
Every stem cell therapy begins with a scientific discovery. Researchers identify specific stem cells that have the potential to repair, regenerate, or replace damaged tissues. These cells might come from sources such as bone marrow, adipose tissue, or umbilical cord blood, each with unique properties suited for different medical applications.
Once identified, these cells undergo extensive preclinical testing. Preclinical research often involves laboratory experiments and animal studies to evaluate the cells’ behavior, effectiveness, and safety. Scientists study how stem cells differentiate into specific tissues, how they interact with the body, and whether they trigger unwanted immune responses or complications. This stage is crucial for designing therapies that are not only effective but also safe for human use.
Rigorous Clinical Trials
If preclinical results are promising, the therapy progresses to clinical trials. These trials occur in multiple phases, each with specific goals:
- Phase 1 trials focus primarily on safety. A small group of volunteers receives the stem cell therapy to identify potential side effects and determine safe dosage ranges.
- Phase 2 trials assess the therapy’s effectiveness while continuing to monitor safety. Researchers expand the participant pool and refine the treatment protocol based on early results.
- Phase 3 trials involve a larger and more diverse group of participants, comparing the stem cell therapy to standard treatments or placebos. Successful completion of this phase is a strong indicator that the therapy is both safe and effective for widespread use.
Throughout clinical trials, regulatory oversight is essential. Agencies like the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) review data rigorously to ensure that therapies meet strict safety and efficacy standards.
Regulatory Approval and Manufacturing
Once clinical trials demonstrate safety and effectiveness, companies can apply for regulatory approval. This process is meticulous, requiring submission of comprehensive data on preclinical studies, clinical trials, and manufacturing processes.
Manufacturing stem cell therapies for real-world use presents unique challenges. Stem cells are living products that must be handled under controlled conditions to maintain their quality, potency, and viability. Advanced facilities use specialized equipment and protocols to produce therapies consistently and safely. Strict quality control ensures that every batch meets the high standards required for patient treatment.
Integration into Clinical Practice
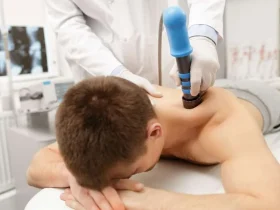
After regulatory approval, the therapy can be integrated into clinical practice. Medical professionals receive training on proper administration, patient selection, and monitoring to ensure optimal outcomes. Hospitals and specialized clinics implement protocols to deliver stem cell therapies safely and effectively.
Patients interested in these treatments often consult specialized centers that provide comprehensive care, combining clinical expertise with state-of-the-art technology. For those exploring options, learning about reputable providers is key. For example, stem cell treatment with auragens.com offers patients access to advanced therapies in a clinical setting backed by experienced professionals.
Ongoing Research and Real-World Data
Even after a therapy reaches the market, research continues. Scientists collect real-world data to monitor long-term outcomes, track rare side effects, and explore new applications. This ongoing research can lead to improvements in existing treatments or the development of entirely new therapies.
The pathway from research to real-world use ensures that stem cell therapies are safe, effective, and accessible to patients who need them most. While the journey is long and complex, it represents a critical process in bringing innovative medical solutions from the lab bench to the bedside.





































Leave a Reply